- Autor:
- Eduardo Anitua
- Lozano-Sanroma J
- Barros A
- Alcalde I
- Alvarado-Villacorta R
- Sánchez-Ávila RM
- Queiruga-Piñeiro J
- Cueto LF
- Merayo-Lloves J
Efficacy and Safety of Plasma Rich in Growth Factor in Patients with Congenital Aniridia and Dry Eye Disease
Abstract
Congenital aniridia is a rare bilateral ocular malformation characterized by the partial or complete absence of the iris and is frequently associated with various anomalies, including keratopathy, cataract, glaucoma, and foveal and optic nerve hypoplasia. Additionally, nearly 50% of individuals with congenital aniridia experience symptoms of ocular dryness. Traditional treatment encompasses artificial tears and autologous serum. This study aimed to assess the effectiveness and safety of using platelet rich in growth factors (PRGF) plasma in patients with congenital aniridia and ocular dryness symptoms.
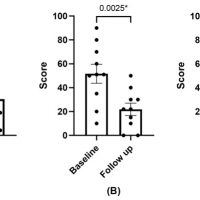
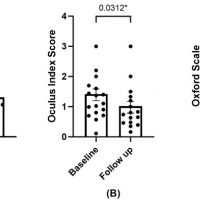
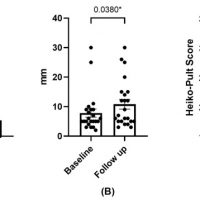
Methods: The included patients underwent two cycles of a 3-month PRGF treatment. At 6 months, symptomatology was evaluated using the OSDI and SANDE questionnaires, and ocular surface parameters were analyzed.
Results: The OSDI and SANDE values for frequency and severity demonstrated statistically significant improvements (p < 0.05). Ocular redness, corneal damage (corneal staining), and tear volume (Schirmer test) also exhibited statistically significant improvements (p < 0.05). No significant changes were observed in visual acuity or in the grade of meibomian gland loss.
Conclusions: The use of PRGF in patients with congenital aniridia and ocular dryness symptoms led to significant improvements in symptomatology, ocular redness, and ocular damage. No adverse effects were observed during the use of PRGF.

 English
English
 Français
Français
 Deutsch
Deutsch
 Italiano
Italiano
 Português
Português